For global healthcare research leads, the start of 2026 marks more than just a new calendar year; it marks the official commencement of the 15th Five-Year Plan (2026–2030) and a finalized regulatory framework that fundamentally alters the "Safety Profile" of China-based studies.
While we have previously explored the Digitalization of Healthcare, the 2026 landscape is now defined by a "Zero-Tolerance" approach to data and ethics.
The SAMR 2026 Anti-Bribery Mandate
In late 2025, the State Administration for Market Regulation (SAMR) finalized the Compliance Guidelines for Healthcare Companies to Prevent Commercial Bribery Risks. This is a landmark shift because it explicitly categorizes "Academic Visits" and "Consulting Services," the very heart of qualitative research, as high-risk areas.
The Change:
Regulators no longer distinguish between "marketing" and "market research" if the payment flow is opaque. Under the 2026 Cybersecurity Law (CSL) amendments, immediate fines of up to RMB 2 million can be issued for procedural violations without a prior warning period.
The PIPL "Certification" Era
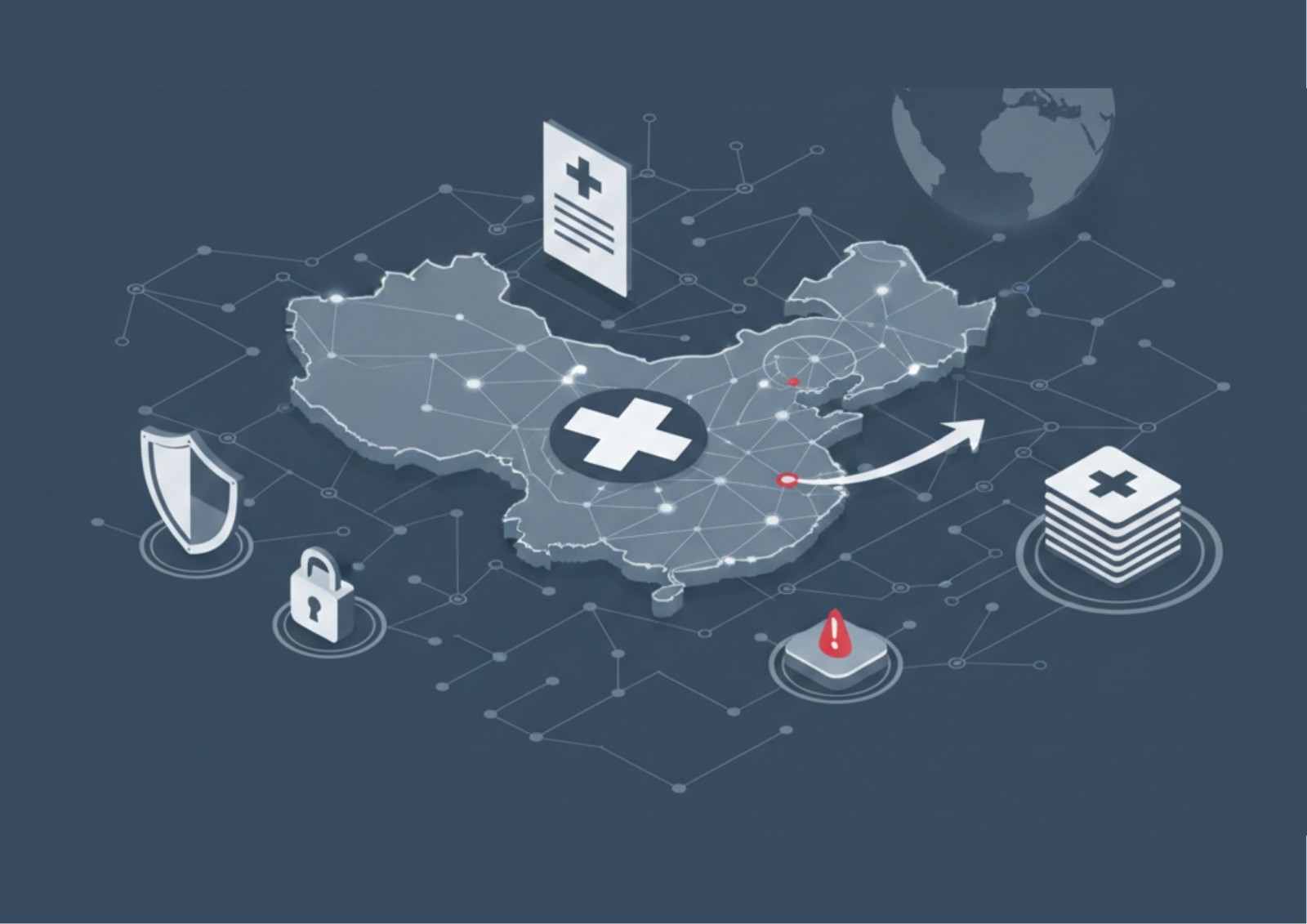
As of January 1, 2026, the Measures for the Certification of Outbound Transfer of Personal Information are fully in effect. For agencies without a China-based entity, the "Standard Contract" is no longer the only route.
The New Pathway:
The PIP Certification now offers a unified, 3-year compliance mechanism for multinational groups. However, it requires a rigorous Personal Information Protection Impact Assessment (PIPIA) that must be kept on file for at least three years.
The Healthcare Sensitivity:
Because medical data is categorized as "Sensitive Personal Information" under PIPL, the threshold for mandatory Security Assessments by the CAC is significantly lower. Transferring sensitive data for even 10,000 individuals now triggers the highest level of state scrutiny.
The "Value-Based" Research Shift
The continued expansion of Volume-Based Procurement (VBP) in 2026 has pushed hospital margins to the limit. As we noted in our Tiered City Analysis, this has made hospitals and HCPs far more selective about who they engage with.
• The Research Pivot: Insight leads are moving away from "Brand Awareness" studies (which feel too commercial) toward "Value-Based Integrated Care" studies, aligning with the "Health-First" strategy of the 15th Five-Year Plan.
The Youli Strategy: Your Compliance "Safe Harbor"
At Youli, we didn't wait for 2026 to change our methods. We have built a strategy that turns these policy hurdles into a competitive advantage for our partners:
- Fair Market Value (FMV) Audit: We utilize a 2026-updated FMV calculator that accounts for seniority, specialty, and tiered-city location, ensuring every honorarium payment stands up to SAMR scrutiny.
- Audit-Ready PIPIA: Every healthcare project we run is accompanied by a PIPIA document that satisfies both the PIPL Certification and Standard Contract requirements.
- Local "Cleaning" Nodes: To mitigate the risks of "Sensitive Data" export, we de-identify HCP and patient data on local, secured China-based servers before it ever leaves the mainland.
Get in touch today
_1769067678558.jpg)